Semi Conductors:
If a substance whose sensitivity lies in between conductors and insulators is known as semi conductors and insulators is known as semiconductor. It is important to note that the sensitivity does not alone decide whether a substance is a semi conductor or not.For example,an alloy may be prepared whose sensitivity falls within the range of semiconductors but this cannot be regarded as a semiconductor.In fact,there are number of peculiar properties which distinguish whether a material is a semiconductor,conductor or an insulator.
Properties of a semi conductor:
The following are the properties of semi conductor:
1.They have sensitivity less than insulators and less than conductor.
2.The resistance of semiconductor decreases with the increase in temperature and vice versa i.e., they have negative temperature coefficient of resistance.
3.When suitable metallic impurity like arsenic,gallium,etc., is added to a semiconductor,then the current conducting properties changes appreciably.This is the most important property.
Formation of chemical bonds in semi conductors like Germanium and silicon:
Semi conductors like Germanium and silicon have crystalline structure. Germanium and silicon are tetravalent materials i.e.,each has four valence electrons in outermost shell.
1.Bond formation in Germanium:
Germanium has thirty two electrons,twenty eight electrons are tightly bound to the nucleus while remaining four revolve in the outermost orbit.These four electrons are known as valence electrons.Nucleus with twenty eight tightly bound electrons forms the positive core of the atom.
Let us consider the case when the two atoms of Germanium are brought close to each other.Now,the positive core of one atom interacts with one of the valence electrons of the other atom and the two electrons are shared from the other atom and the two electrons from the other atom.These two such electrons form an electron pair as shown below.,
When the attractive force is balanced by the repulsive force between two electrons of positive core forms a covalent bond.The covalent bonds binds between the adjacent atoms.As shown in figure each circle represents ionic core of germanium atom which carries a positive charge +4e,where e represents the magnitude of charge of an electron.
Each of the four valence electrons associated with an atom is represented by a solid circle.The electron pair or covalent bond is represented by a solid small circle.Since the valence electron binds an atom to the adjoining one,the valence electrons themselves get tightly bound to the nucleus.Hence inspite of availability of four valence electrons,the crystals,the crystal has low conductivity.
2.Bond formation in Silicon atom:
The explanation for silicon is similar to that of Germanium.The below fig., shows the bond formation in silicon.
Effect of temperature in semi conductors:
At a very low temperature(say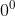 K),the semi conductor crystal behaves as a perfect insulator since the covalent bonds are very strong and no.of free electrons are available.In terms of energy band,there is large gap between valence band and conduction band.Hence the valence band is completely filled no electron can reach the conduction band to become a free electron.
K),the semi conductor crystal behaves as a perfect insulator since the covalent bonds are very strong and no.of free electrons are available.In terms of energy band,there is large gap between valence band and conduction band.Hence the valence band is completely filled no electron can reach the conduction band to become a free electron.
At room temperature,some of the covalent bonds are broken due to the thermal energy supplied to the crystal.Due to breaking of bonds,some electrons become free which were engaged in the formation of these bonds.This is shown in fig 2.,
The electron disloged from the covalent bond becomes free to wander in a random fashion throughout the crystal. The absence of electron in the covalent bond is represented by a small circle.This empty place or vacany left behind the crystal structure is called a hole.Since an electron has a unit negative charge,the hole.Since an electron has a unit negative charge,and the hole carriers has a unit positive charge.The importance of hole is that it may serve as a carrier of electricity in the same general way as free electron.
Energy band diagram of germanium:
The energy band diagram of Germanium is as shown below fig.3.,
The forbidden gap for germanium is 0.7 eV. In germanium even at room temperature when a covalent bond is broken,the electron has acquired sufficient energy to jump across the energy gap from valence band to conduction band.For each electron liberation in conduction band a positive charge is created in the valence band.Hence,this hole represents the missing of electron in covalent bond.
Each of the four valence electrons associated with an atom is represented by a solid circle.The electron pair or covalent bond is represented by a solid small circle.Since the valence electron binds an atom to the adjoining one,the valence electrons themselves get tightly bound to the nucleus.Hence inspite of availability of four valence electrons,the crystals,the crystal has low conductivity.
2.Bond formation in Silicon atom:
The explanation for silicon is similar to that of Germanium.The below fig., shows the bond formation in silicon.
 |
| fig.1, bond formation in silicon |
At a very low temperature(say
At room temperature,some of the covalent bonds are broken due to the thermal energy supplied to the crystal.Due to breaking of bonds,some electrons become free which were engaged in the formation of these bonds.This is shown in fig 2.,
The electron disloged from the covalent bond becomes free to wander in a random fashion throughout the crystal. The absence of electron in the covalent bond is represented by a small circle.This empty place or vacany left behind the crystal structure is called a hole.Since an electron has a unit negative charge,the hole.Since an electron has a unit negative charge,and the hole carriers has a unit positive charge.The importance of hole is that it may serve as a carrier of electricity in the same general way as free electron.
Energy band diagram of germanium:
The energy band diagram of Germanium is as shown below fig.3.,
 |
| fig.3., energy band diagram of Ge |
Mechanism of conduction in electrons and holes:
When the electrons are liberated on breaking of covalent bonds,they move randomly through the crystal lattice.These free electrons are not attracted by the nuclei of the atoms at the same time not even repelled by the electrons bound by covalent bonds because their electrical effects are completely engaged in maintaining the covalent bonds.
When an electric field is applied to crystal lattice these free electrons have a steady drift opposite to the direction of applied field.This constitute electric current.We know that when a covalent bond breaks,a hole is created.For one free electron(electron is removed or set out),one hole is created,as a result,electron-hole pair is created by thermal energy.These holes move through the crystal in a random fashion like liberated electrons.When an external electric field is applied,the holes drift in the direction of applied field.Thus they constitute electric current.The current conduction by hole is as shown below.,
Now let us understand the mechanism of free electron from point A to D:
Generally,the valence electron of a covalent bond becomes free electron by application of thermal energy or any external electric field.Now at point A,let us apply thermal energy.On the application of thermal energy,the electron at point A becomes free electron.This creates a hole in the covalent bond at A.It should be noted that there is a strong tendency for a semiconductor crystal to form covalent bond.Therefore,a hole attracts an electron from the neighboring atom.Now,a valence electron(say at B) from near by covalent bond,comes to fill in the creation of holes at A.This results in the creation of hole at B.This hole has thus effectively shifted from A to B.Subsequently,let this hole move from B to C,from C to D and so on. This movement of holes in the absence of an applied field is random.
When an electric field is applied,the hole drift along the applied field.This constitute the hole current.The below figure., gives the energy band description of hole current.
let us assume that an electron leaves the valence band to enter into conduction band.This leaves a vacancy in valence band at L.Now,the valence electron at M comes to fill the hole at L.This process is repeated from valence electron like M,N,P. Thus the valence electrons move along the path P,N,M,L while the holes move along the path L,M,N,P.When the movement of valence electron takes place from one covalent bond to another,it constitute hole current.
When an electric field is applied to crystal lattice these free electrons have a steady drift opposite to the direction of applied field.This constitute electric current.We know that when a covalent bond breaks,a hole is created.For one free electron(electron is removed or set out),one hole is created,as a result,electron-hole pair is created by thermal energy.These holes move through the crystal in a random fashion like liberated electrons.When an external electric field is applied,the holes drift in the direction of applied field.Thus they constitute electric current.The current conduction by hole is as shown below.,
Now let us understand the mechanism of free electron from point A to D:
Generally,the valence electron of a covalent bond becomes free electron by application of thermal energy or any external electric field.Now at point A,let us apply thermal energy.On the application of thermal energy,the electron at point A becomes free electron.This creates a hole in the covalent bond at A.It should be noted that there is a strong tendency for a semiconductor crystal to form covalent bond.Therefore,a hole attracts an electron from the neighboring atom.Now,a valence electron(say at B) from near by covalent bond,comes to fill in the creation of holes at A.This results in the creation of hole at B.This hole has thus effectively shifted from A to B.Subsequently,let this hole move from B to C,from C to D and so on. This movement of holes in the absence of an applied field is random.
When an electric field is applied,the hole drift along the applied field.This constitute the hole current.The below figure., gives the energy band description of hole current.
let us assume that an electron leaves the valence band to enter into conduction band.This leaves a vacancy in valence band at L.Now,the valence electron at M comes to fill the hole at L.This process is repeated from valence electron like M,N,P. Thus the valence electrons move along the path P,N,M,L while the holes move along the path L,M,N,P.When the movement of valence electron takes place from one covalent bond to another,it constitute hole current.
Note:
The basic reason for current flow is the presence of holes in the covalent bonds.Hence it is more reasonable to consider it as a hole current rather than electron current.
Carrier recombination and generation:
In an intrinsic semiconductor,the number of free electrons equals the number of holes.The reason is that,a hole is created only by removing an electron from covalent bond.Thus the electrons and holes are generated in pairs.The free electrons and holes move randomly within the crystal lattice.In such a random motion,there is always a possibility that a free electron may have an encounter with a hole.Even though there is less possibility that a free electron meeting a hole,but still they recombine to re-establish the covalent bond.
2.The energy so released may in turn be re-absorbed by another electron to break its covalent bond.In this way new electron hole pair is created.
Classification of Semi Conductors:
Semi Conductors are classified as two types:
1. Intrinsic semi conductor
2.Extrinsic semiconductor
1.Intrinsic semi conductors:
A semi conductor in an extremely pure is known as intrinsic semiconductor.In intrinsic semiconductor the number of electrons is always equal to number of holes in conduction band .As the electrons reaching the conduction band due to thermal excitation leave the number of vacancies or holes in valence band such that, the number of free electrons becomes equal to the number of holes.
Examples: Silicon,Germanium
Mechanism of intrinsic semi conductors on the application of electric field:
When an external electric field is applied across an intrinsic semiconductor,the conduction in semi conductors is due to both free electrons and holes.The free electrons in conduction band move towards the positive terminal of the battery while the holes in valence band move towards the negative terminal of the battery i.e., the electrons and holes move in opposite direction.The total current inside the semiconductors is thus the sum of currents due to free electrons and holes.
b)P-type extrinsic semi conductors:
Mechanism:
When a small amount of trivalent impurity is added to a pure crystal during the crystal growth,the resulting crystal during the crystal growth,the resulting crystal is called a p-type extrinsic semiconductor.Let us consider the case when trivalent Boron is added to pure Germanium crystal.
This is because the three valence electrons of boron atom form covalent bonds with the valence electrons of germanium atom.In,the fourth covalent bond ,only germanium atom attribute one valence electron and there is a deficiency of one electron which is called a hole.In otherwords,we can say that the fourth bond is incomplete;being short of one electron. Therefore for each boron atom added,one hole is created.A small amount of boron provides millions of holes.The energy band description of P-type semi conductor is shown in above fig b.,
However,there are few electrons in conduction band,due to the thermal energy associated with room temperature.In the incomplete covalent bond,the remaining four electrons of germanium atom tries to form a covalent bond.Actually it does so by taking the advantage of thermal motion.It steals an electron from the neighboring germanium atom.Due to this stealing action,a hole is created in the adjacent atom.This process continues and the hole moves about in a random way due to its thermal effects.Impurity atoms that contributes hole in this manner are termed as acceptor because they accept electrons from germanium atoms.Since,the current carriers are positively charged,this type of semi conductors is called a p-type semi conductor.
The following points are to be remembered for p-type semi conductors:
1.In p-type semi conductor materials,the majority carriers are positive holes while the minority carriers are electrons.
2.The p-type semi conductor remains electrically neutral as the number of mobile holes under all conditions remains equal to the number of acceptors.
3.When an electric field is applied across p-type semi conductor,the current conduction is predominantly by holes.Here the holes are shifted from one covalent bond.As the holes are positively charged,they are directed towards negative terminal and constitute the hole current.The hole current flows more slowly than the electron current in N-type semi conductor.
4.In p-type semi conductivity,the valence electrons move from one covalent bond to another covalent bond unlike the n-type where current conduction is by free electrons.
The basic reason for current flow is the presence of holes in the covalent bonds.Hence it is more reasonable to consider it as a hole current rather than electron current.
Carrier recombination and generation:
In an intrinsic semiconductor,the number of free electrons equals the number of holes.The reason is that,a hole is created only by removing an electron from covalent bond.Thus the electrons and holes are generated in pairs.The free electrons and holes move randomly within the crystal lattice.In such a random motion,there is always a possibility that a free electron may have an encounter with a hole.Even though there is less possibility that a free electron meeting a hole,but still they recombine to re-establish the covalent bond.
2.The energy so released may in turn be re-absorbed by another electron to break its covalent bond.In this way new electron hole pair is created.
Classification of Semi Conductors:
Semi Conductors are classified as two types:
1. Intrinsic semi conductor
2.Extrinsic semiconductor
1.Intrinsic semi conductors:
A semi conductor in an extremely pure is known as intrinsic semiconductor.In intrinsic semiconductor the number of electrons is always equal to number of holes in conduction band .As the electrons reaching the conduction band due to thermal excitation leave the number of vacancies or holes in valence band such that, the number of free electrons becomes equal to the number of holes.
Examples: Silicon,Germanium
Mechanism of intrinsic semi conductors on the application of electric field:
When an external electric field is applied across an intrinsic semiconductor,the conduction in semi conductors is due to both free electrons and holes.The free electrons in conduction band move towards the positive terminal of the battery while the holes in valence band move towards the negative terminal of the battery i.e., the electrons and holes move in opposite direction.The total current inside the semiconductors is thus the sum of currents due to free electrons and holes.
It may be noted that the current in the external wires is not only due to electrons as the holes reach the negative terminal B,the electrons reaching here combine with the holes.Thus holes are destroyed.At,the same time,the loosely held electrons near the positive terminal A are attracted away from their atoms into positive terminal.Now,much holes are created which again drift towards negative terminal B.
2.Extrinsic semiconductors:
At room temperature,the intrinsic semiconductors has little current conduction capability. In order to use semiconductors in electronic devices,its conduction properties should be increased.The electrical properties of intrinsic semi conductor can be increased by adding some impurity in the process of crystallization.The added impurity will be very small of the order of one atom per million atoms of pure semi conductors.The semi conductor thus formed by addition of impurity is known as extrinsic semi conductors.The process of adding impurities to semi conductor is known as doping.
Types of doping materials:
Usually the doping material is either pentavalent or trivalent atoms.
1. Pentavalent doping:
The pentavalent doping atom is known as donor atom because it donates one electron to the conduction band of pure semiconductor as it has five valence electrons.
Examples: Bismuth,Arsenic,Antimony,Phosphor
2.Trivalent doping:
The trivalent doping atom is called as acceptor atom because it accepts one electron from semi conductor atom as it has three valence electrons.
Examples: Gallium,Indium,Aluminium,Boron
The doping materials are called impurities because they alter the structure of pure semi conductor crystals.
Classification of extrinsic semi conductors:
Depending upon the type of impurity added,the extrinsic semi conductors can be divided into two classes:
a) N-type extrinsic semi conductors
b) P-type extrinsic semi conductors
a) N-type extrinsic semi conductors:
Mechanism:
When a small amount of penta valent impurity is added to a pure semiconductor crystal during the crystal growth,the resulting crystal is called as N-type extrinsic semi conductors.The below fig., represents a crystal lattice with one germanium atom displaced by Arsenic atom and energy band diagram of N-type extrinsic semi conductor.
As shown in fig., the arsenic atom fits in the germanium crystal in such a way that its four valence electrons form covalent bonds with the germanium atom.The fifth electron of arsenic atom is not covalently bonded but it is loosely bound to the parent arsenic atom.This available electron serves as carrier of current.The amount of energy needed to detach this valence electron from impurity atom is of the order of only 0.01eV for Ge and 0.05eV for Si using As impurity.Since this energy is very small,therefore it results in thermal agitation at room temperature.Such a liberated valence electron is then free to move randomly in crystal lattice in the same way as the free electrons move in an intrinsic semi conductor.Although each arsenic atom provides only one free electron,yet an extreme amount of arsenic impurity provides enough atoms to supply millions of free electrons.
In addition to the electrons and holes availability in pure germanium,doping of antimony greatly increases the number of conduction electrons.Thus,the concentration of electrons in conduction band is increased and hence exceeds the number of holes in valence band.It should be noted that,by giving away or donating one of its valence electron,the arsenic atom becomes positively charged ion.It cannot take part in conduction because it is firmly fixed in crystal lattice.The fifth left over valence electron is not accommodated in valence band.This electron travels to the conduction band.
The following points are to be remembered for N-type semi conductors:
1.In N-type semi conductor material,there will be excess of electrons.Here the number of holes are small when compared to the parent intrinsic semi conductor because there are large number of electrons available to fill up the vacancies.Thus in N-type semi conductor,the electrons are the majority carriers while positive holes are minority carriers.
2.Although N-type semi conductors has excess of electrons but it is electrically neutral.This is due to the fact that the electrons are created by the addition of neutral pentavalent impurity atom i.e., there is no addition of negative charges or the positive charges.The extra electrons are free electrons and increases the conductivity.
3.When N-type semi conductors is placed between the two electrodes and an electric filed is applied to it as shown below.,
Here the excess electrons donated by impurity atoms will travel towards the positive impurity atoms will travel towards the positive electrodes.This constitute the electric current.This type of conductivity is called negativity or N-type conductivity because the current flow through the crystal is due to free electrons(negatively charged particles).It may be noted that this conduction is just as in ordinary metals like copper.
Mechanism:
When a small amount of trivalent impurity is added to a pure crystal during the crystal growth,the resulting crystal during the crystal growth,the resulting crystal is called a p-type extrinsic semiconductor.Let us consider the case when trivalent Boron is added to pure Germanium crystal.
This is because the three valence electrons of boron atom form covalent bonds with the valence electrons of germanium atom.In,the fourth covalent bond ,only germanium atom attribute one valence electron and there is a deficiency of one electron which is called a hole.In otherwords,we can say that the fourth bond is incomplete;being short of one electron. Therefore for each boron atom added,one hole is created.A small amount of boron provides millions of holes.The energy band description of P-type semi conductor is shown in above fig b.,
However,there are few electrons in conduction band,due to the thermal energy associated with room temperature.In the incomplete covalent bond,the remaining four electrons of germanium atom tries to form a covalent bond.Actually it does so by taking the advantage of thermal motion.It steals an electron from the neighboring germanium atom.Due to this stealing action,a hole is created in the adjacent atom.This process continues and the hole moves about in a random way due to its thermal effects.Impurity atoms that contributes hole in this manner are termed as acceptor because they accept electrons from germanium atoms.Since,the current carriers are positively charged,this type of semi conductors is called a p-type semi conductor.
The following points are to be remembered for p-type semi conductors:
1.In p-type semi conductor materials,the majority carriers are positive holes while the minority carriers are electrons.
2.The p-type semi conductor remains electrically neutral as the number of mobile holes under all conditions remains equal to the number of acceptors.
3.When an electric field is applied across p-type semi conductor,the current conduction is predominantly by holes.Here the holes are shifted from one covalent bond.As the holes are positively charged,they are directed towards negative terminal and constitute the hole current.The hole current flows more slowly than the electron current in N-type semi conductor.
 |
| fig., p-type conductivity |
4.In p-type semi conductivity,the valence electrons move from one covalent bond to another covalent bond unlike the n-type where current conduction is by free electrons.
Mobile charge carriers and immobile ions:
1.For N-type semi conductors:
Consider the case of N-type semi conductor.This is formed by adding pentavalent antimony,bismuth,phosphorus etc., to intrinsic semi conductor.This results in an additional electrons to the crystal lattice.The electrons move away from the parent atoms and the atoms acquire positive charges.A positively charged atom is known as donor ion and its position is fixed in the crystal lattice.It is important to mention here that the donor ion(immobile ion) cannot take part in conduction.Therefore,N-type semi conductor may be regarded as material consisting of donor ions i.e., immobile ions and electrons.
 |
| fig.,represents immobile ions of p-type and n-type materials respectively |
The p-type semi conductors are formed by the addition of trivalent atoms such as aluminium,gallium,etc.,This results in an additional holes in the crystal lattice.The holes move away from the parent atoms and the atoms acquire negative charges.A negatively charged atom is known as acceptor atom and its position is fixed in the crystal lattice.The holes move away from the parent atoms and the atoms acquire negative charges. A negatively charged atom is known as acceptor atom and its position is fixed in the crystal lattice.The holes move away from the parent atoms and the atoms acquire negative charges.A negatively charged atom is known as acceptor atom and its position is fixed in the crystal lattice.The acceptor ion(immobile ion) cannot take part in conduction.Therefore P-type semi conductor may be regarded as a material consisting of acceptor ions i.e.,immobile ions and holes i.e., mobile charge carriers.
Mass Action Law:
In an intrinsic semi conductors,the electrons and holes are always present in equal concentrations.When N-type impurity is added to an intrinsic semi conductor,the concentration of free electrons is increased or concentration of hole decreased below the intrinsic value.Similarly,the addition of P-type impurity increases the hole concentration or the concentration of free electrons below the intrinsic value.
Under thermal equilibrium,the product of concentration n of free electrons and concentration p of holes is constant and is independent of the amount of doping by donor and acceptor impurities.This is known as mass-action law.Thus,
where
Charge densities in semi conductor:
1.Intrinsic semi conductor:
In case of an intrinsic semi conductor,
where n and p are the electron and hole concentration respectively and  is the intrinsic concentration.
is the intrinsic concentration.
The densities of free electrons and holes are further interrelate by the law of electrical neutrality.Let  be the concentration of donor atoms.As the donor atoms are ionized at room temperature,there will be
be the concentration of donor atoms.As the donor atoms are ionized at room temperature,there will be  immobile positive charge per cubic meter contributed by donor ion.Now the total positive charge density is
immobile positive charge per cubic meter contributed by donor ion.Now the total positive charge density is  .
.
Let  be the concentration of acceptor atom.In this case,there will be
be the concentration of acceptor atom.In this case,there will be  immobile negative charges per cubic meter.The total negative charge density is thus
immobile negative charges per cubic meter.The total negative charge density is thus 
Since the semi conductor is electrically neutral,the magnitude of positive charge density must be equal to magnitude of negative charge density.
Thus,
2.Extrinsic semi conductor:
N-type semi conductor:
In N-type semi conductor,there is no acceptor doping i.e.,  . Moreover, the number of electrons is much greater than the number of holes(n>>p).In this case,
. Moreover, the number of electrons is much greater than the number of holes(n>>p).In this case,
since n>>p,
Thus in N-type material,the free electron concentration is approximately equal to the density of donor atom.To distinguish the concentration of free electrons in n and p regions,we add subscript n for an n-type and p for p-type.
To distinguish the concentration of free electrons in N and P regions,we add subscript n for an N-type and p for P-type.Thus
Now the concentration
P-type semi conductor:
In P-type semi conductor,there is no donor doping i.e.,
Now the concentration of electrons in p-type material is
and
Electrical properties of semi conductor materials:
Here we shall study four electrical properties of semi conductors:
1.Conductivity
2.Intrinsic concentration
3.Energy Gap
4.Mobility
1.Conductivity:
Semi conductor is a bipolar device.In this material,conduction is done by electrons as well as holes.The conductivity of semi conductor is obtained by summing the conductivity of electrons and the conductivity of holes.The conductivity of the semi conductors due to electrons in conduction band is given by
The conductivity of the semi conductors due to holes in conduction band is given by
The total conductivity is given by
let us study the conductivity of semi conductors in intrinsic and extrinsic semi conductors
(i) Intrinsic semi conductors;
In case of intrinsic semi conductors (Ge or Si) the number of conduction electrons is equal to the number of holes i.e
where
therefore the conductivity
(ii)Extrinsic semi conductors:
a) Conductivity of N -type semi conductor:
In case of N -type semi conductor the electron concentration is far greater than the hole concentration i.e.,n>>p.That means the hole concentration is neligibly small in comparsion to electron concentration.The electron concentration is now represented by
b)Conductivity of P -type semi conductor:
In case of P-type semi conductor the electron concentration is neligably small in comparsion to hole concentration i.e.,p>>n.Let the hole concentration is represented by  i.e., concentration of acceptor atom.Hence the conductivity of P-type semi conductor is given by
i.e., concentration of acceptor atom.Hence the conductivity of P-type semi conductor is given by
2.Intrinsic Concentration:
The intrinsic concentration increases with increasing temperature.As the temperature increases,the conductivity also increases.It has been established that the intrinsic concentration varies with the temperature according to the relation,
where
K is Boltzmann constant
3.The Energy Gap:
The energy gap is equal to the difference of energy levels between conduction band and valence band of semi conductor crystal structure.It is observed that energy gap decreases with increase in temperature.
At any temperature T,the energy gap is expressed by the following relation:
where,- for Silicon energy at 0k is 1.21eV
- for Germanium energy at 0k is 0.785eV
Therefore,
>The energy gaps of Si and Ge
The mobility of charge carriers varies over a temperature range of 100 to 400 degrees kelvin.
I have created this blog for educational purpose,so for that i have written the content by referring many books,web pages.I have also uploaded google images and you tube videos for the better understanding of concept and I would also like to inform you that I am not responsible for the ads which are being posted in my blog.












No comments:
Post a Comment